The Large Intestine
The large intestine comprises the cecum, colon, rectum, and anal canal and is responsible for minerals, water, and any remaining nutrients to be absorbed, forming feces from the undigestable meal components (2/3 of mass) and bacteria (1/3 of mass). The cecum is a pocket connected to the end of the ileum via the ileocecal valve that acts as a receiving reservoir for the large intestine. Extending from the cecum is the appendix, which is sometimes located off the ascending colon, not part of the intestinal tract but instead considered as gut-associated lymphoid tissue and believed to contain a sampling of the microbiota in case of depletion so that the large intestine could be repopulated. The large intestine accounts for approximately 1/3 of the digestive tract length and is subdivided into the ascending, transverse, and descending colons. Unlike the small intestine, the large intestine plays little role in the absorption of macronutrients; however, the gut microbiota ferments unabsorbed and indigestible food components producing volatile short-chain fatty acids (SCFA), which are absorbed and utilized by the epithelial cells for energy. Initially, the meal propagates from the cecum through the ascending colon to the transverse colon, supported by the mesentery by peristalsis to the descending colon. The rectum, ascending, and descending colons are retroperitoneal organs fixed in location as they lack a complete covering of the peritoneum (membrane lining of the abdominal cavity), while the cecum, appendix, transverse, and sigmoid colons are intraperitoneal organs and mobile as they are surrounded by peritoneum. The internal diameter decreases progressively through each segment.Meal exits the small intestine via the ileocecal valve and moves from the cecum up the transverse colon by peristalsis.
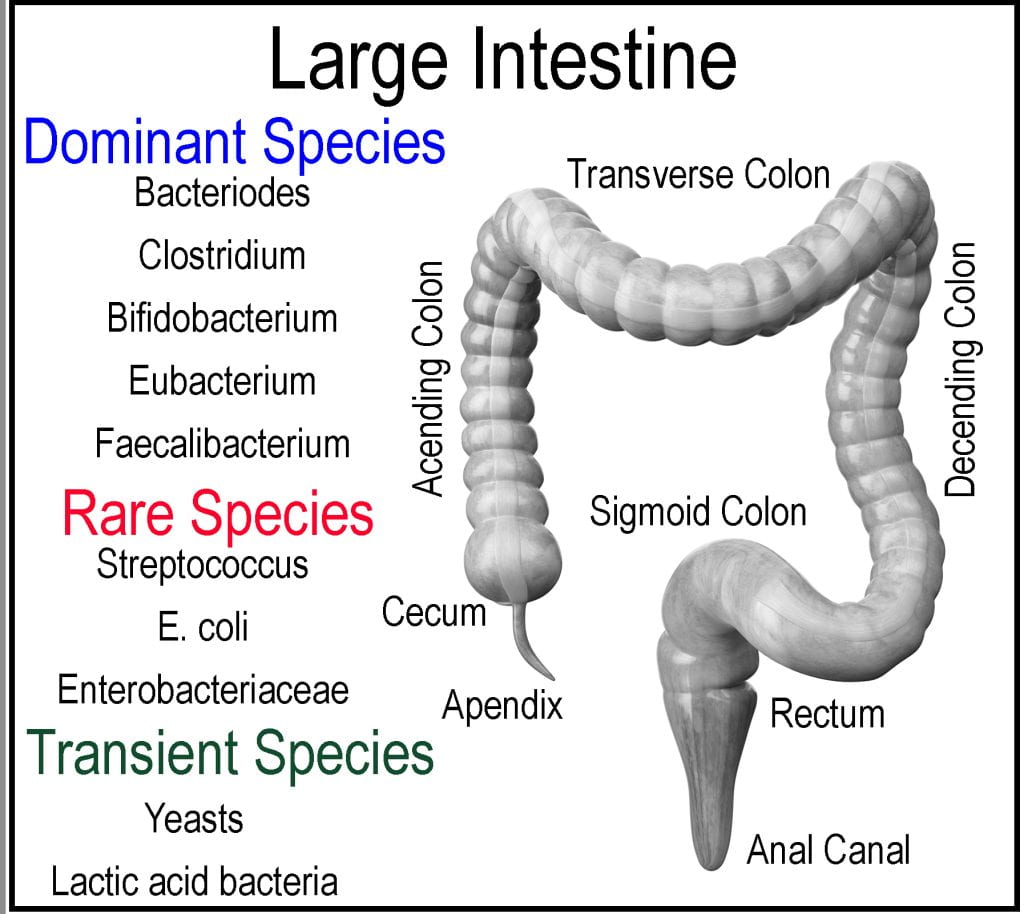
Instead of being a long tube similar to the small intestine, each section of the large intestine is segmented, similar to a series of small pouches called haustra. Three bands of smooth muscle (Taeniae coil) connected at the cecum extend the entire length of the large intestine to the rectum. However, the muscle is shorter than the length of the large intestine it connects, causing haustra to form. The mesentery supplies the entire length of the colon blood supply through two mesenteric arteries, while the lymphatic systems connect each segment via a series of lymph nodes and to the nervous system via the sympathetic and parasympathetic systems. The lining of the large intestine differs from the small intestine as its primary focus is not to absorb nutrients but instead to absorb excess water.
The colon lining secretes mucosa and is invaginated, unlike the villa appendages, which contain colonic crypts that are microscopic, muscle-lined, tube-like invaginations. Material entering the ascending colon is a liquid, but excess water is slowly absorbed, causing the stools to solidify as it transits the descending colon. Sodium ions are pumped into the intercellular space by cells in the intestinal lining to raise the osmolarity of the intercellular fluid, creating a hypertonic fluid and an osmotic pressure gradient leading to osmosis of water through the tight junctions and adjacent cells.
Microbiome and Its Metabolites
The large intestine houses the gut microbiome, which via microbial fermentation, exerts beneficial (e.g., breakdown of undigestable components to products that are absorbed, production of B-vitamines (thiamine (B1) and riboflavin (B2)) by E. coli, and lactic acid bacteria producing other nutraceuticals) and undesirable effects (e.g., microbes produce toxic sulfur compounds, carcinogens, gases). The microbiome metabolizes many more compounds than human digestive enzymes as the microbiome contains 30 X (750,000) of the genes found in the human genome, and less than 300,000 of those genes are shared by greater than 50% of individuals underlining the incredible diversity of the gut microbiome (Shreiner, 2015). The microflora ferments non-glycemic carbohydrates (fiber) to produce additional energy sources such as short-chain fatty acids (SCFA)). SCFAs are produced in the colon by microbial fermentation; butyrate is an essential energy substrate for cellular metabolism in the colonic epithelium (Tremaroli, 2012). In addition to being fermented, non-glycemic carbohydrates bind bile, facilitating its excretion. By limiting bile reuptake, the liver requires cholesterol to make new bile, causing an overall cholesterol reduction. The gut microflora is extremely complex, containing hundreds of phylotypes, including phyla from Bacteroidetes, Actinobacteria, Firmicutes, Fusobacteria, and Alphaproteobacteria (Eckburg, 2005).
The gut microflora is established starting in the first few days of life, varying tremendously through the life cycle (Penders et al., 2006). Infancy establishment of the gut microbiota is determined depending on the mode of delivery, where those born by cesarean have lower Bifidobacteria and Bacteroides but higher C. difficle than vaginally born infants. Next, the type of feeding shows formula-fed infants are more often colonized with E. coli, C. difficile, Bacteroides, and Lactobacilli. Finally, gestational age, hospitalization and antibiotic use also influenced the microbiome composition (Penders et al., 2006). The three dominant phyla include Bacteroidetes, Firmicutes & Actinobacteria, and they form a complex environment where they are influenced by the presence of other phyla and their metabolites. The intricate interplay these populations have on forming symbiotic and antagonistic between each other, and the host constantly evolves with age, highlighted by the changing ratio between Firmicutes/Bacteroidetes(F/B ratio) from infancy to adulthood to the elderly. After the biome is established in infancy, the F/B ratio is near zero, indicating a low Firmicutes count compared to Bacteroidetes, which increases and peaks during adulthood before declining again (Mariat, 2009). A healthy microbiome is essential for human health as the microbiome plays a symbiotic role in human health. However, altered gut microbiota can also cause dysbiosis, a microbial imbalance or maladaptation that manifests as inflammatory bowel disease, asthma, and hypertension, as well as disrupts mood and behavior due to hormones produced by the biome involved in hormone signaling (Nicholson, 2012).
An example of the utilization of non-digestible substrates that we lack digestive enzymes to hydrolyze includes a class of soluble fiber, xyloglucans, and hemicelluloses found in the primary cell wall of vascular plants. The gut microbiota is integral to host digestion and nutrition as some phyla generate nutrients from indigestible substrates such as xyloglucan. Microbial digestion of xyloglucans was recently mapped to a single locus of Bacteroides, and while rare, it was a rare trait among Bacteroides, 92% of individuals have at least one rare Bacteroides species capable of digesting xyloglucans (Shreiner, 2015). Considering the diversity and size of the microbiome, their genes encode many different hydrolytic enzymes produced throughout digestion. A few examples include nitroreductases (converts R-NO2 to R-NH2), sulfatases (hydrolyzes R-O-SO3- to R + S042-), azoreductases (Converts N=N to HN-NH), all produced by the microbiome. There is still much unknown regarding the microbiome-host relationship and the potential impact on digestion. The role of the microbiome in assessing the safety of food additives thought inert is just being invested as there is potential to be metabolized into toxic substrates. Some bacteria also produce the hormones (PY) involved in the neural signing of satiety and may play a role in obesity.
Digestive Enzymes of the Colon
While no endogenous digestive enzymes are produced in the large intestine encoded in the human genome, the microbiota contributes enzymes relevant to human nutrition as they can alter undigestable polysaccharides, polyphenols and B and K vitamins. Polysaccharides, especially soluble and non-soluble fiber and any residual starch, are fermented by saccharolytic bacteria producing short-chain fatty acid (SCFA) and other metabolites (lactate, succinate, etc.). Acetate (C2), propionate (C3), and butyrate (C4) are the most abundant SCFAs produced, and each plays a relevant role in human health, albeit complex, providing an energy source for the epithelial lining of the colon (colonocytes), altering gluconeogenesis, and potentially regulating appetite. These and other metabolites produced by saccharolytic bacteria cross-feed other bacteria; this interplay of availability of nutrients and waste products establishes a unique ecosystem in the ascending, descending, and transverse colons. Acetate production is common among commensal bacteria; Firmicutes tend to produce butyrate, while Bacteroides produce propionate. Depending on the host’s microbial population, gas production is variable during anaerobic fermentation as some species do not produce gas (lactobacilli and bifidobacteria). Proteins available in the colon that remain undigested, or contained in the mucin, or sloughed off the intestinal cell, or digestive enzymes are broken down into amino acids and shorter peptides by proteolytic bacteria to produce branched short-chain fatty acids (used as a biomarker) and gases H2, C02, H2S
Works Cited
Eckburg, P.K. et al., 2005, diversity of the human intestinal microbial flora. Science. 308:1635-1638.
Nicholson, J.K. et al., 2012. Host-Gut Microbiota Metabolic Interactions.Science. 336: 1262-1267.
Mariat, D., et al., 2009. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. 9: 123.
Penders, J., et al., 2006. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 118: 511-521.
Shreiner, A.B., et al., 2015. The Gut Microbiome in Health and Disease. Current Opinion in Gastroenterology. 31:: 69-75.
Tremarolin, V., et al., 2012. Functional Interactions Between the Gut Microbiota and Host Metabolism. Nature. 489: 242-249.


