Solutions
Recall that chalcogen atoms, or group VI hydrides, include oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po). Due to lone electron pairs in their valence shells, these molecules lack symmetry when covalently bound to two hydrogen atoms (i.e., H2O, H2S, H2Se, and H2Te). However, water will be the obvious focus herein; hydrogen chalcogenides are all polar molecules. Hydrogen chalcogenides, H2X, are triatomic molecules with a chalcogen, bent molecular configuration, and the presence of lone electron pairs that causes a deviation in the tetrahedral bond angle from 109.5 in methane (CH4) to 104.5 in the bent configuration for water and to 92.1o for H2S, 91.0o for H2Se and 90.0o for H2Te—attaining the ability to act as a solvent able to solvate a solute. The bent structure and uneven charge distribution give rise to the dipole moment of water, which it uses to solvate the molecule by aligning its charge to both negative ions and polar molecules. Water is an excellent solvent, capable of solvating numerous charged and polar molecules. However, hydrophobic molecules such as vegetable oil do not form solutions and are discussed later under dispersions.
Because of its polarity, water forms electrostatic interactions with other polar molecules and ions, and solutions contain two or more types of molecules that, when combined, are homogenously (evenly) distributed across the system. Each solute (e.g., NaCl, glucose, water-soluble vitamin) in the solution, at low concentrations, only interacts with water molecules and cannot form interactions with other solutes as this results in aggregation and often precipitation. Electrostatic interactions between water and the solute occur because water aligns itself according to the solute’s charge (d– of oxygen aligns with positively-charged solute while the d+ of water’s hydrogens aligns with negatively charged solutes). At low solute concentrations, there is a greater abundance of water than solute; when this is the case, water forms a hydration shell around the solute forming a clear solution.
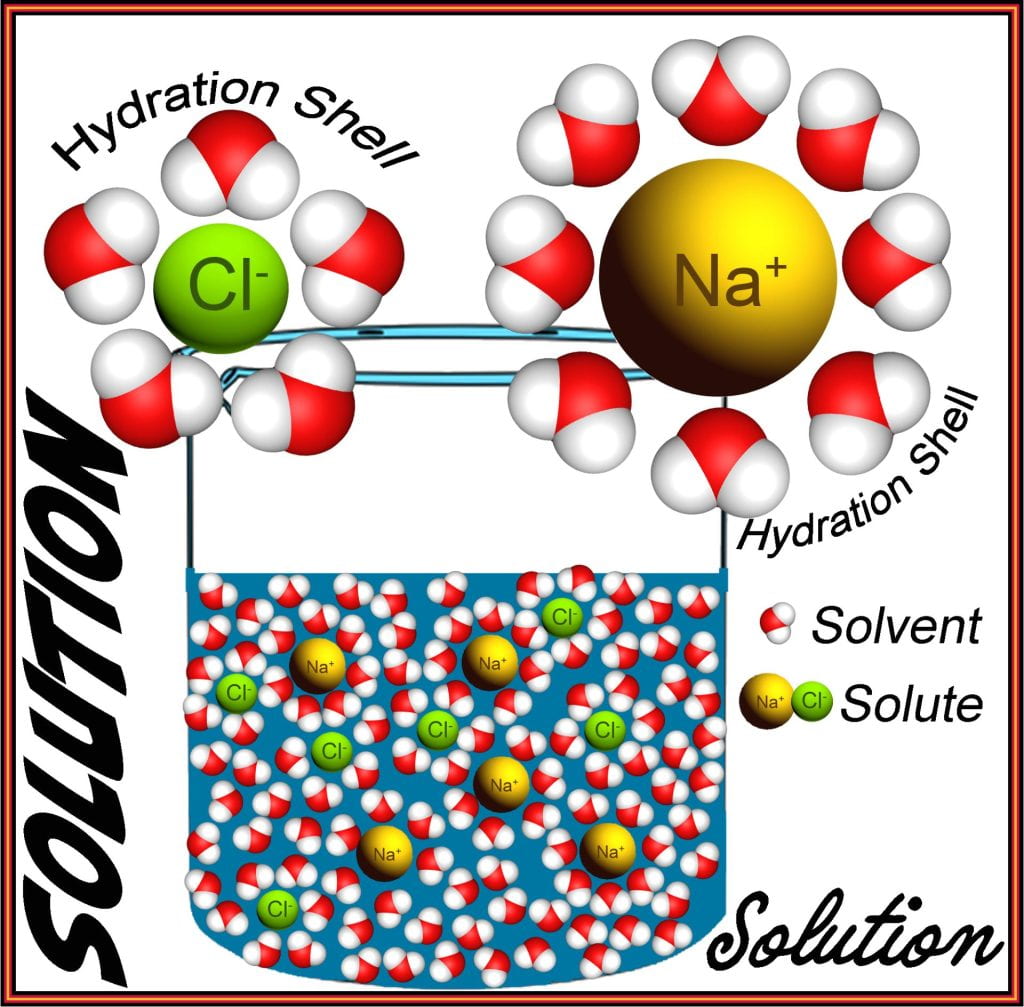
When the attractive forces holding the solute particles together are weaker than their water attraction, solvation occurs when water molecules completely hydrate the surface, forming a hydration shell. The hydration shell containing a solute molecule can diffuse readily, keeping apart by Brownian motion. Solvent molecules exceed solute molecules in a solution. All solvent-solute combinations have a solute concentration limit to their solubility; solubility is also highly sensitive to temperature, where increasing temperature increases the solubility threshold allowing more solute to be dissolved. Solutions remain clear with no particles larger than the diffraction limit of light.
Colligative Properties
Osmotic pressure is the external pressure required to prevent the net movement of a solvent across a semipermeable membrane. Osmosis spontaneously occurs from a region of high water potential (lower solute concentration) to a region of low water potential (higher solute concentration), causing diffusion of solvent molecules through a selectively-permeable membrane in the direction that dilutes the solute. Osmotic pressure is the minimum pressure needed to prevent the inward flow of solvent across a semipermeable membrane in the presence of a concentration gradient.

Plant and mammalian cell walls act as semipermeable membranes, and three scenarios emerge when placed in a solution: 1) the osmolality of dissolved solute is the same inside and outside the cell, the solution is isotonic, and there is no net movement of water across the semipermeable membrane; 2) the osmolality of dissolved solute is greater inside than outside the cell, the solution is hypotonic, and there is a net movement of, and gain of, water into the cell; and, 3) the osmolality of dissolved solute is greater outside than inside the cell, the solution is hypertonic, and there is a net movement and loss of water out of the cell. Plant and blood cells residing in a hypertonic solution relative to the cytoplasm cause the net water loss from the cell; the cell shrinks and then puckers (undulating surface), losing its turgor pressure and becoming flaccid. If there is a large enough gradient, the cell is plasmolyzed, causing the cell membrane to disintegrate. Conversely, if a biological cell is in a hypotonic solution relative to the cytoplasm, the cell interior accumulates water, and water flows across the cell membrane into the cell, causing expansion and increasing turgor pressure. An obvious illustration of osmotic pressure in action is highlighted when putting limp celery into the water; the higher concentration of solutes in the vegetable establishes a concentration gradient and net movement of water into celery, increasing the turgor pressure and crispness of celery.
Vapor Pressure
When a liquid is contained in a closed system (e.g., a sealed container impermeable to gas) with an air headspace, a fraction of molecules will escape the liquid phase evaporating into the gas phase of the headspace. Molecules constantly exchange between the phase liquid-vapor phases establishing an equilibrium vapor pressure above the liquid. Equilibrium vapor pressure is temperature dependent and affected by the cohesive attractive forces holding liquid molecules together. Hereafter the focus on liquids is the triatomic molecule – water, a hydrogen chalcogenide (H2X)) with strong cohesion due to strong intermolecular hydrogen bonds. High cohesion results in stronger attractive forces in liquids which require greater kinetic energy to escape the liquid phase lowering the vapor pressure.
Adding solute to a liquid, in this case, water, establishes a new equilibrium vapor pressure for the solution that depends on the osmolality. The addition of solute at low concentrations >~10% follows Raoult’s Law “the partial pressure of each component of an ideal mixture of liquids is equal to the vapor pressure of the pure components multiplied by its mole fraction in the mixture.” The added solute molecules occupy space, effectively reducing the surface area of the air-water interface, where evaporation occurs, by the mol fraction of the solute and solvent. Raoult’s law for a single component in an ideal solution (e.g., sugar water or salt water solutions) has a partial pressure (pi) equal to the equilibrium vapor pressure (pi*) of the pure component, i (e.g., water), and its mole fraction of the solution.
pi=pi*ci

Boiling Point Elevation
The boiling point of a liquid occurs at the temperature where the vapor pressure of the liquid is equal to the vapor pressure above. In an open system, at atmospheric pressure (1 atm), water boils at 100 oC. If the pressure above the liquid is reduced under vacuum, water boils at T< 100 oC, while if pressure is applied, water boils at T>100 oC. Establishing why water boils at 100 oC is important to understand how the boiling point elevates when a solute such as salt or sugar is added. Added solute molecules occupy space, effectively reducing the surface area of the air-water interface, where evaporation occurs, by the mol fraction of the solute and solvent. This results in a lower partial pressure for the solution than pure water, which means there is a larger difference in the partial and atmospheric pressure and more energy or higher temperature is needed to boil a sugar or salt solution than pure water.
Boiling point elevation is essential in food science. Candy thermometers measure boiling point elevation, a useful parameter, perhaps most so in confectionery science, which is the science of sugar and fat. As a sugar water solution is boiled, water is removed as vapor, increasing the sugar concentration of the solution and osmolarity, establishing a higher boiling point. Based on Raoult’s Law, boiling point elevation determines the type of structure the solution will form upon cooling. At 110oC, the sugar solution is termed ‘thread,’ where the sugar concentration reaches ~85%. Threads are viscous fluids such as maple and corn syrups, and when the syrup is slowly added to cold water, it forms a liquid thread that will not ball up. At a boiling point of 113-115 oC, the ‘soft-ball’ stage increases in sugar concentration to 85% and forms fudges and fondants; when dropped in cold water, it forms a ball and, when removed, it flattens.

The ‘firm-ball‘ stage, at 87% sugar, boils between 118-121oC and is used to make caramels, forming a firm ball in cold water that retains its shape but remains malleable. The ‘hard-ball’ stage reaches 92% sugar and boils between 121-129oC, characterized by forming a hard-ball when the syrup is dropped into cold water; once removed, it will not flatten, but it remains soft enough to deform under pressure. The hard-ball stage is the endpoint for nougat, gummies, marshmallows and rock candy. The ‘soft-crack‘ stage increases sugar concentration to 95% with a boiling point between 132-143oC.
Adding soft-crack to cold water solidifies into flexible, not brittle threads as they deform (bend) before failing (break). Soft-crack candies include saltwater taffy and butterscotch. ‘Hard-crack‘ candy, including toffee, brittle and lollipops, is boiled until 132-143oC, reaching 98-99% sugar concentration. When dropped into cold water, the sugar solution forms hard, brittle threads that are very brittle and not malleable. Beyond the hard-crack stage, at a temperature >160oC, boiling point elevation is no longer affected by the concentration as very little water is present (>99% sugar); at this point, sugar begins caramelizing, which is the condensation of sugar monomers into polymers of caramelans (C24H36O18), caramelens (C36H50O25), and caramelins (C125H188O80).
Freezing Point Depression
Freezing point depression from the liquid to solid state operates using the same principle, except now it is a difference in the chemical potential between the solution and solid. The chemical potential is the potential energy stored and released from a molecule going between the liquid and solid states. The presence of a solute reduces the chemical potential changing the solidification (crystallization) temperature similar to boiling point elevation, just in the opposite direction. Freezing point depression of sodium chloride to ice water allows the water to cool below 0oC without freezing; however, freezing point depression is important for most frozen foods, especially ice cream. If a soluble sugar solution is cooled and allowed to reach thermodynamic equilibrium, T2, at each temperature, it will reach and follow the solution ice phase boundary T2 to T3. As the solution cools, water crystallizes into ice; as this occurs, the water becomes unavailable to solubilize sugar, effectively increasing the sugar concentration in the unfrozen solution. At this point, the saturation limit of the solution is exceeded, and as it continues to cool, the viscosity of the unfrozen phase kinetically impeeds water molecules from the solution phase to the ice crystal surface, and crystallization stops. At this point, T4, continued cooling occurs without further crystallization, causing an enormous increase in the unfrozen phase viscosity impeding molecular motion and forming a glass.



