Caramelization
While there are many important reactions involving simple mono and disaccharides, reactions involved in sugar browning occur via caramelization and the Maillard Research, both are essential in developing desired organoleptic properties of foods when applying high heat (cooking), resulting in a sweet, nutty flavor and brown color. Caramelization occurs in both reducing and non-reducing sugars, and the temperature needed to initiate the reaction is dependent on the molecular mass of the sugar (fructose (110 oC) < glucose (150 oC) < galactose (160 oC) < sucrose (170 oC) < maltose (180 oC) < lactose (203 oC)). Oxidation of sugars occurs during the decomposition, and inversion hydrolyzes the glycosidic disaccharide bonds in sucrose or maltose, creating fructose and glucose and establishing an equilibrium between the anomeric and cyclic sugars. The rate of decomposition is dependent on pH, and caramelization occurs slowest at neutral acidity (pH ~ 7) and accelerates under acidic (pH < 3) and basic (pH > 9) conditions. Enolization rearranges the carbonyl oxygen atom on the sugar molecule to become an enol (alcohol attached to a carbon adjacent to a double-bond carbon).
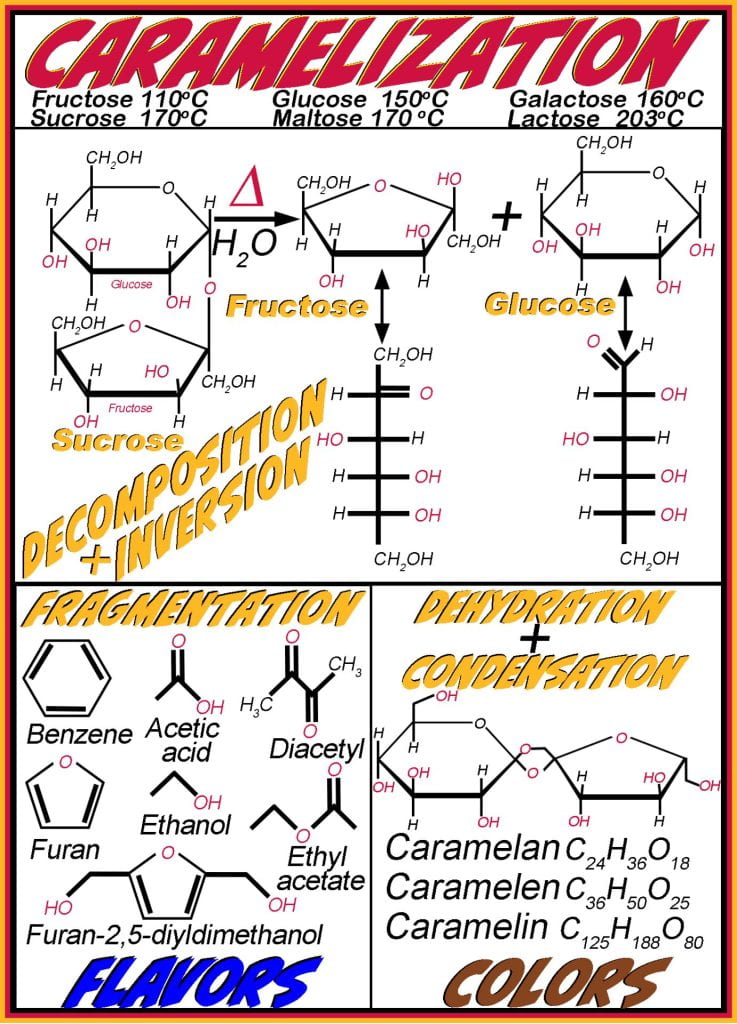
After sucrose undergoes inversion and decomposes into glucose and fructose, condensation reactions occur near pH 3 and 9, increasing the average molecular mass. The individual sugars lose water following the condensation step, causing them to condense before undergoing aldose to ketose isomerization. Continued heating removes additional water, causing dehydration reactions, and once water content begins to decline, the molecules begin to fragment into smaller volatile molecules such as benzene, acetic acid, diacetyl, furans, ethanol, ethyl acetate and furan-2,5-diyldimethanol. In addition to those listed, hundreds of new aromatic compounds formed during caramelization result in complex flavors and aromas that differ depending on pH, temperature and processing time. For example, diacetyl imparts a butter/butterscotch flavor, which furans are nutty. Other important flavor compounds include hydroxymethylfurfural (HMF), hydroxyacetylfuran (HAF), hydroxydimethylfuranone (HDF), and dihydroxydimethylfuranone (DDF).
These small molecules are polymerized alongside sugars and contain significant amounts of unsaturation, which is essential for developing the desired brown color of caramel. The brown colors are a result of the formation of caramelans (C24H36O18), caramelens (C36H50O25), and caramelins (C125H188O80). The shorter carmelanes are more water soluble and commonly used beverages, such as Pepsi and Coke, while caramels and caramels are used more often in confectionaries. If the reaction is not carefully monitored and controlled, it will progress until it lacks sweetness and become bitter.
Maillard Reaction
The Maillard reaction is non-enzymatic browning that occurs between amino acids and reducing sugars, providing the distinctive color and flavor of seared meat, fried dumplings, bread crumb, coffee, chocolate, toasted marshmallows, and many other cooked foods.
The hydrolysis reaction during the Maillard reaction liberates water; thus, a high water activity environment inhibits the Maillard Reaction. The reducing sugar in the linear form has an exposed reactive carbonyl group that interacts with the nucleophilic amino group on an amino acid at the terminal end of the protein forming the shiff base or N-substituted glycosylamine. This process accelerates in an alkaline environment because the amino groups are not neutralized. Once the shift base is formed between the reducing sugar and amino group, unstable glycosylamine undergoes Amadori rearrangement, forming ketosamines, 1,2-enaminol, 2,2-enaminol, and the amadori product. Small molecules such as acetic and formic acid are also produced, and other short-chain hydrolytic fission products are formed. After amadori rearrangement, Strecker degradation hydrolyzes the amino acid forming dicarbonyls and aldehydes, and at the same time, the amino acid fragments undergo fission, dehydration and degradation, resulting in small volatile compounds such as acrolein and acrylamide and other aromatic and flavor compounds. The small amino acid sugar fragments then undergo cyclization, providing flavor compounds ranging from meaty to toasted to buttery, polymerizing to produce brown melanoidin pigments.



