Fermentation
Biological preservation prolongs shelf-life by altering intrinsic food properties (pH, water activity, bacteriocins (nicin)) using either endogenous or added beneficial microorganisms and the metabolites (e.g., organic acids, ethanol, CO2) they produce during fermentation. At the same time, the application for fermentation extends well beyond bio preservation and is used in synthesizing recombinant rennet used in cheese making and recombinant human insulin.

As it applies to preserving fermented foods, microorganisms can include mold or fungus, yeast and bacteria, subdivided based on the primary organic acid metabolite (acetic, lactic and propanoic acid). The most commonly used are yeasts in bread and alcohol production and lactic acid bacteria in salami, yogurt, cheese, kimchi and pickles. Fermentation is also essential in treating silage and haylage to prevent pathogens and reduce the likelihood of rumen acidosis, a metabolic condition in cattle. Additionally, native microflora, at the time of harvest, may also be used in fermentation, as is the case with cocoa beans, where they are wrapped in banana leaves and left to ferment for 6 to 10 days. During fermentation, the seed coat and germ layers are destroyed, and the breakdown products provide the characteristic taste of cocoa.
Anaerobic fermentation breaks carbohydrates down into simple sugars, which are utilized for energy (ATP production) and, in the process, release metabolites of ethanol, CO2 and organic acid, each of which, as they accumulate, inhibit other microorganisms from proliferating. Additionally, when organic acids are produced, the food is acidified to a pH~4, acting as an intrinsic barrier to microbial spoilage. Also, the substrate reduction (sugars) impedes further microbial growth, and the high concentration of probiotic (bacteria used in fermentation) inhibit colonization of new microorganisms. Besides alterations in flavor and shelf-life, fermentation reduces the food glycemic index because bacteria consume sugars and increase micronutrient density, including B vitamins and other probiotic effects (e.g., inflammation reduction, leaky gut and colitis).
Bacterial Fermentation
Fermentation of milk is used to manufacture cheese, yogurt, and kefir. For yogurt, a binary live probiotic culture containing Lactobacillus delbrueckii ssp. bulgaricus and/or ssp lactis and Streptococcus thermophilus are required for fermentation. Both organisms break down the disaccharide lactose into its corresponding monosaccharides, glucose and galactose, and through glycolysis, both convert glucose into pyruvate and lactic acid. Only Lactobacillus can break galactose into lactic acid, while Streptococcus does not utilize galactose.
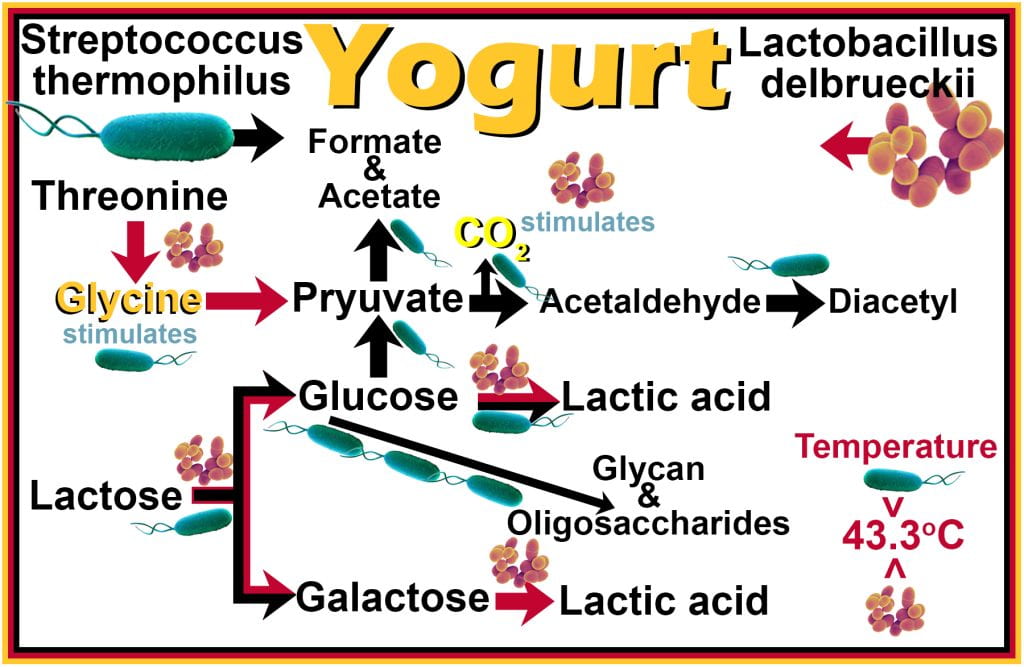
During fermentation, a symbiotic relationship is established where Lactobacillus utilizes threonine and liberates glycine, which stimulates the growth of Streptococcus; conversely, the CO2 produced by Streptococcus, while breaking pyruvate down into acetaldehyde and diacetyl (buttery flavors), stimulates Lactobacillus growth. Defects occur during yogurt fermentation when one microorganism becomes in excess. For example, too much Lactobacillus makes the yogurt sour with a thin consistency; while excess Streptococcus produces insufficient lactic acid, and the polymers of glycan and oligosaccharides become ropey with a strong buttery flavor. The growth rates of both organisms are maintained optimally at 43.3oC, above which Streptococcus is favored, while below this temperature, Lactobacillus predominates.

Organic acids, produced during fermentation, function when the pH of the food is near or below the pKa of the acid. In the case of yogurt, during fermentation, the pH of milk decreases to between 4.4 and 4.8 as glucose is converted to lactic acid (pKa = 3.8), resulting in approximately 40% of the lactic acid being in the protonated form and 60% being deprotonated. As the lactic acid passes into the microbial cell, the cytoplasm pH is ~6.0, causing lactic acid to deprotonate and acidify the cytoplasm. Using energy (ATP), the microorganism can compensate by pumping excess hydrogen ions out of the cell via efflux pumps.
The microorganisms survive at low concentrations of ~1% but cannot replicate because all available metabolic energy is used to maintain the cytoplasm pH (bacteriostatic). When the concentration of organic acid is higher, ~2%, it accumulates in the cell faster than the efflux pumps can remove, and the cytoplasm acidifies denaturation essential enzyme resulting in cell death (bactericidal).
Yeast Fermentation
Saccharomyces cerevisiae, also termed brewer’s and baker’s yeast, ferments sugar into pyruvate; but unlike bacteria, yeasts release two molecules of CO2 from the pyruvate to form acetaldehyde, which is reduced to ethanol by NADH which is oxidized by regenerating NAD+ required during glycolysis. Many saccharomyces subspecies are used in alcohol fermentation because they are relatively efficient at alcohol conversion and tolerate higher levels of ethanol <~15%, after which yeast is no longer viable; and to obtain beverages with greater alcohol concentrations requires distillation (boiling and recapturing the volatiles and ethanol while removing water). In beer making, Saccharomyces cerevisiae is a top-fermenting yeast that produces ale beer, which is fermented at warm temperatures, 15-25oC. Lager beer uses a bottom-fermenting yeast, Saccharomyces pastorianus (formally carlsbergensis), which undergoes a cold fermentation (lagering) at 10-15oC.
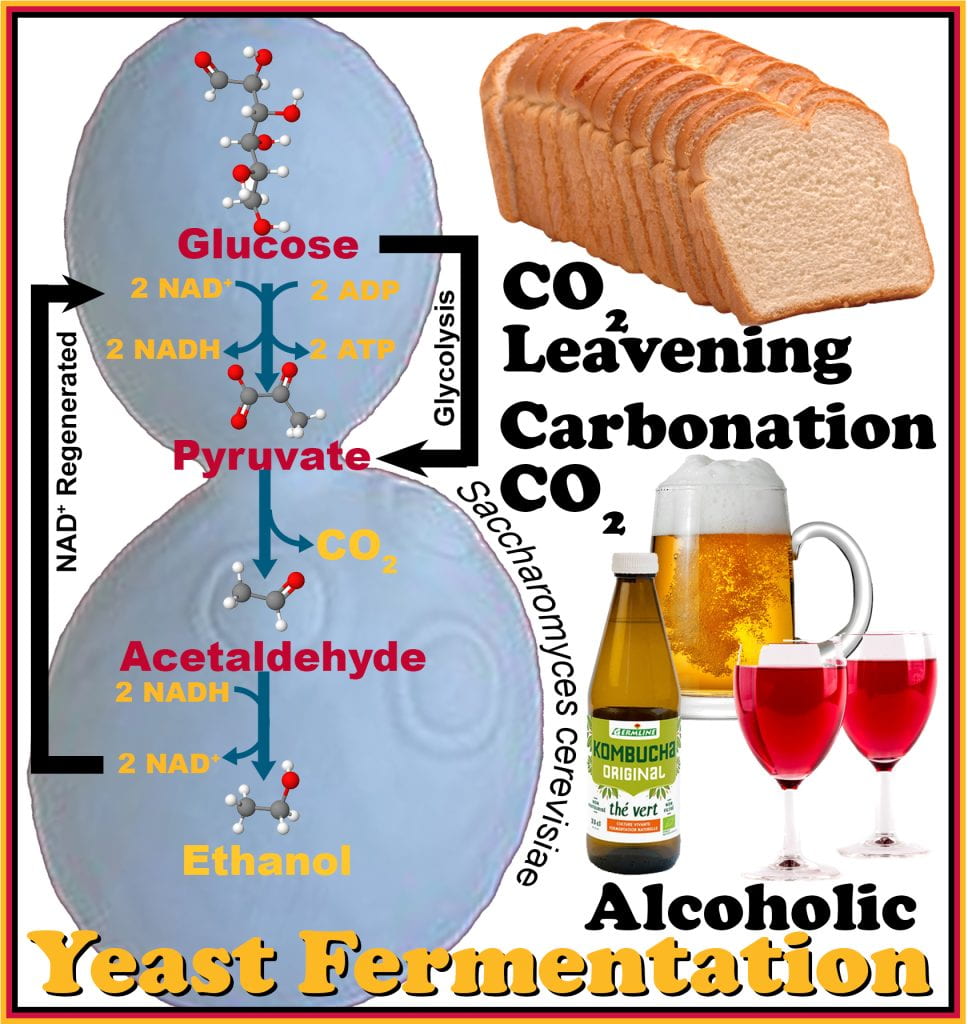
The serving temperature for these beers is opposite to their fermentation temperature; lagers are meant to be served cold (< 4oC), while ales are served near ~10oC. The combined effect of reducing the glucose to support bacterial growth and the presence of alcohol act as intrinsic barriers to growth, where concentrations at 5% impede replication slowing growth rates. Yeast also naturally carbonates alcoholic beverages. Using yeast as a leavening agent (introduce gas) rises dough before baking, and the ethanol produced is quickly evaporated during baking.


